Making blood vessels with 3D-printed sugar
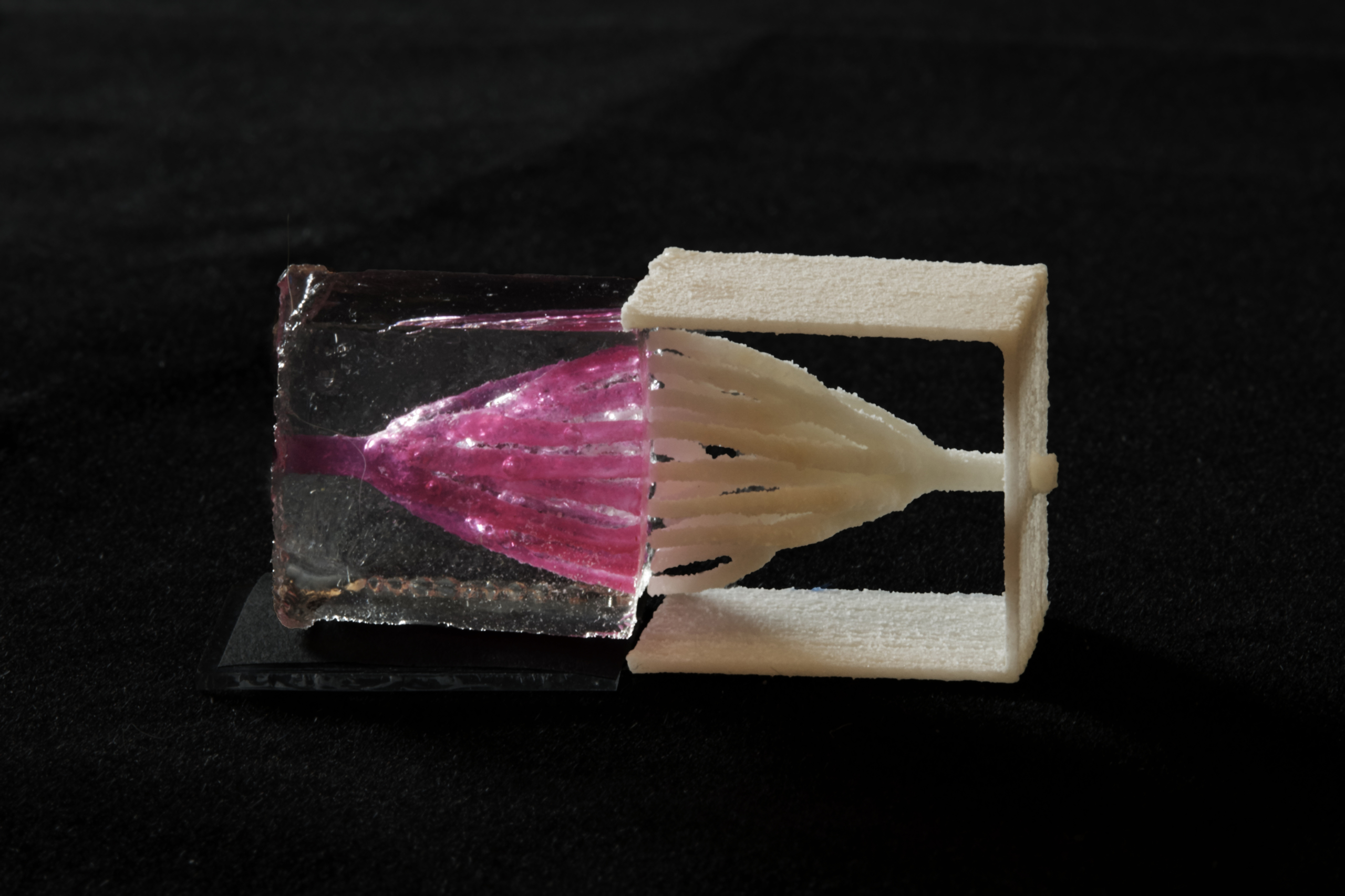
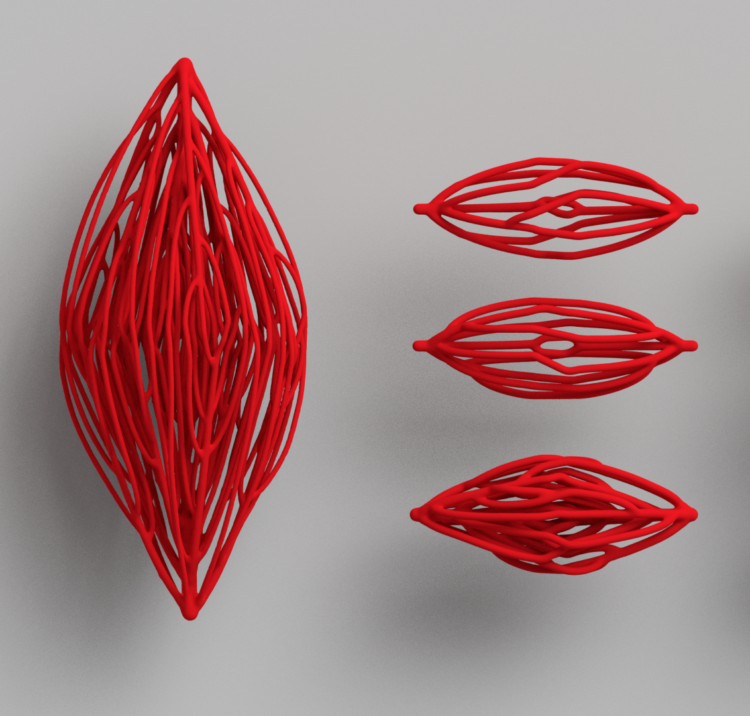
Nervous System collaborated with bioengineers at Rice University to create complex blood vessel networks using generative design and 3D-printed sugar. In a paper published in Nature Biomedical Engineering, we showed that densely packed cells can be kept alive for 2 weeks using these complex blood vessel networks. The research was led by Ian S. Kinstlinger at Jordan Miller’s lab at Rice University.

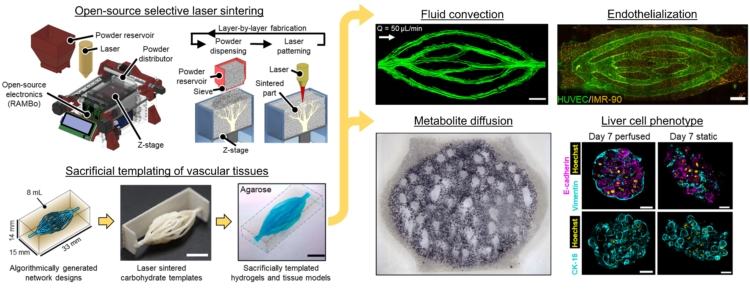
The structures we helped design were 3d-printed in sugar using an open source SLS printer created in Jordan Miller’s lab from a modified laser cutter. After printing, these sugar templates are cast in a mixture of living cells and gel. When the gel has set, the sugar is dissolved and flushed away, leaving the intricate branching network that serves as blood vessels for the living cells.
algorithm development
To create the intricate blood vessel networks studied in this research, we developed a new modification of our Xylem software. Specifically we needed to create networks with an inlet and outlet so blood could be perfused through the structures, coming in on one side and exiting on the other. We call our modification “mutual tree attraction.” The blood vessel network grows from two root points, representing the flow inlet and outlet. In addition to being attracted to source point throughout the gel volume space, the growing trees are also attracted to each other such that they merge upon coming close. After growth the structures are automatically trimmed to remove isolated branches and smoothed to promote good flow. The video below shows the algorithm growing within a 2D ellipse boundary for clarity, however in the research we grew fully 3D structures.
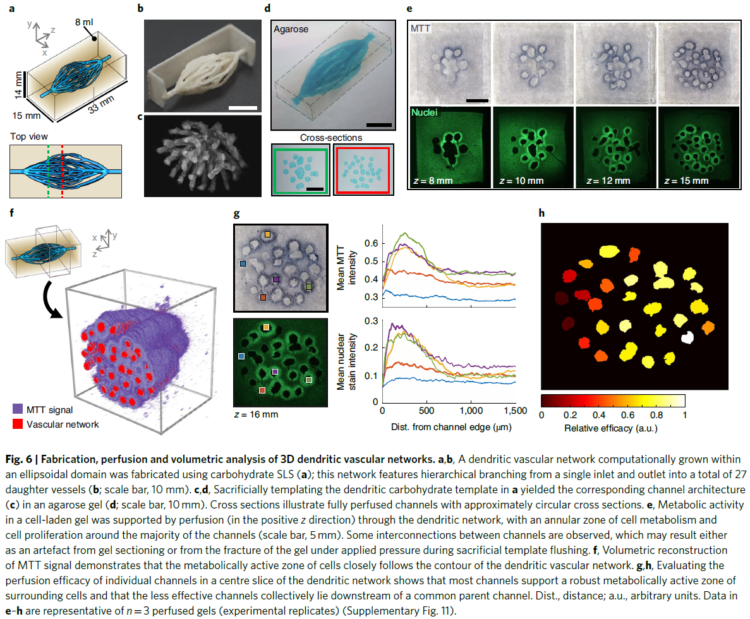
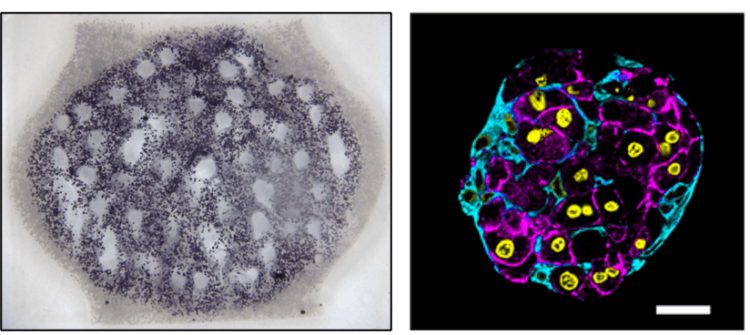
The dendritic networks we generated were extensively studied across several criteria by quantifying fluid flow through the branches and mapping the spatiotemporal dynamics of cell metabolism and proliferation as a function of vascular proximity. Working with Kelly Steven’s group at University of Washington, they studied the ability of these dendritic structures to support the metabolic function of liver cells.
Rice University press release: Laser-welded sugar: Sweet way to 3D-print blood vessels
Nature behind the paper feature: Engineering biomimetic vascular networks
Authors:
Ian S. Kinstlinger, Jordan S. Miller, Gisele Calderon, Karen Vasquez Ruiz, David Yalacki, Palvasha Deme, Kevin Janson, Daniel Sazer and Saarang Panchavati, all of Rice;
Kelly R. Stevens, Sarah Saxton and Fredrik Johansson, both of UW;
Jessica Rosenkrantz and Jesse Louis-Rosenberg of Nervous System;
Karl-Dimiter Bissig of Baylor College of Medicine.
note: I’m publishing this blog post late…the paper came out last summer. But I am just catching up on documentation now after being on maternity leave.





Nervous System Create Complex Blood Vessel Networks with 3D-Printed Sugar – HOLO
[…] Nervous System […]